Table Of Contents
- WHAT IS ULTRAVIOLET C?
- WHAT IS UV ENERGY?
- IS UV-C SAFE FOR HUMANS?
Listen to the article
UV ENERGY
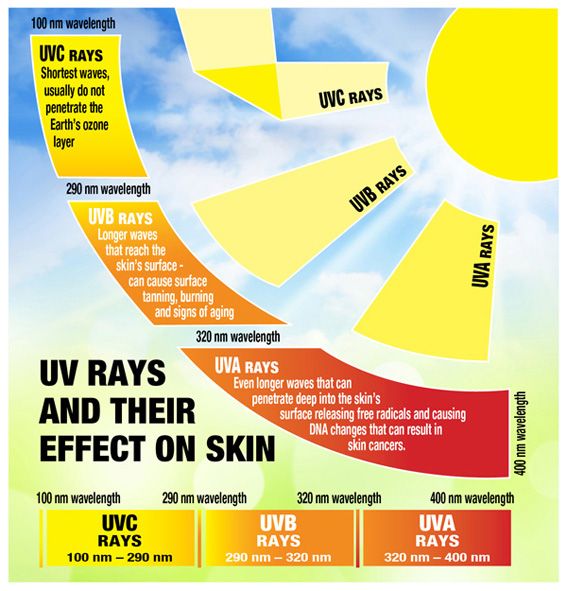
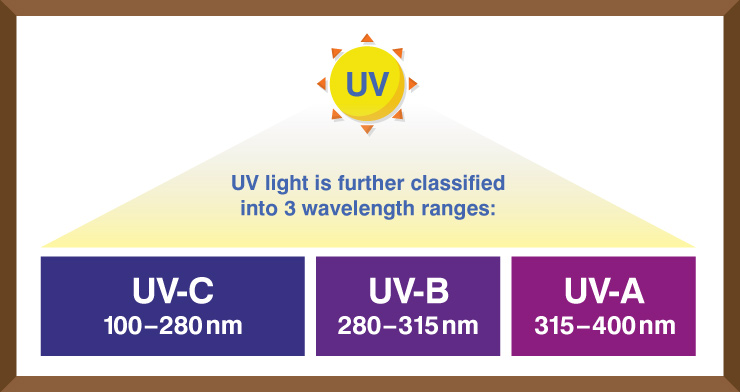
The whole UV range can kill or inactivate numerous microorganism species, keeping them from recreating. UVC vitality at 253.7 nanometers gives the most germicidal impact. The use of UVC vitality to inactivate microorganisms is otherwise called Germicidal Irradiation or UVGI.
WHAT IS ULTRAVIOLET C?
WHAT IS UV ENERGY?
The more significant part of us knows about the unsafe impacts of UV vitality sent by daylight in the UV-An and UV-B frequencies, offering to ascend to UV “burn from the sun” inhibitors or blocking operators, which had found in glasses and salves. We are additionally acquainted with items built to withstand the impacts of UV radiation, such as plastics, paints, and rubbers.
Notwithstanding, unlike the UV-An and UV-B frequencies, the UV-C band has more than double the electron volt vitality (eV) as UV-An, and it is all around retained (not reflected) by natural substances, adding to its danger.
UV ENERGY’S KILLING POWER
UV-C’s germicidal or germ executing impacts have demonstrated. The UV-C frequency owes these harmful impacts to the biocidal highlights of ionizing radiation. All the more essentially, UV-C harms particles in natural frameworks than can temperature alone. Burn from the sun, contrasted with the impression of warmth, is one case of that harm. Burn from the sun is brought about by daylight striking and killing living cells in the epidermis; the subsequent redness from a burn from the sun mirrors the expanded slender activity and bloodstream that permit white platelets to expel the dead cells. UV-C introduction eliminates microscopic organisms and other contamination, causing microbial (and productivity looting) natural contaminants. In the wake of being executed, natural leftovers are dependent upon breaking down.